What is a Colon Transit Study with Transit-Pellets Radiopaque Markers?
A colon transit study with Transit-Pellets is frequently utilized in patients suffering from chronic constipation, chronic diarrhea and/or irritable bowel syndrome (IBS). The purpose of this test is to evaluate the transit time and how fast or slow food/stool travels through the intestines.
The test involves the oral consumption of seven capsules over a six-day period, each containing tiny markers that can be visualized using X-ray imaging. By tracking the progress of the markers through the intestines, the physician can quantify the severity of transit problems and the results can be important in determining the need for additional diagnostic procedures and selecting the appropriate treatment options.
The Transit-Pellets device is a highly effective and non-invasive method for accurately evaluating gastrointestinal function. The device is packed using the Transit-Pellets method, a validated technique for measuring the entire range of colonic transit time (CTT), from fast to slow transit values. This method also has the advantage of requiring only a single X-ray, which reduces patient radiation exposure and keeps costs low.
According to the National Health Service (NHS) in England, consuming the markers is safe and the markers will be eliminated in the feces and can be safely disposed of by flushing them down the toilet (NHS).
Brief History of Tests
The use of ingested radiopaque markers to assess colon transit has been widely accepted since it was first described by Hinton in 1969. The test is sometimes referred to as a “Hintons Method”, but it is also known as the Colon Transit-Pellets test, Colonic Transit Markers Study, Radiopaque Markers Test, Radio-Opaque Markers Test, ROM Test, Metcalf Method, Sitz Marker Study, or Bowel Motility Test. Measuring colonic transit time with radiopaque markers is currently considered the most reliable and safe method for diagnosing and evaluating gastrointestinal disorders in both adults and children.
Transit-Pellets Device Description
The Transit-Pellets device consists of small radiopaque white markers, “pellets”, made of Barium Sulphate (BaSO 4 ) filled silicone inside a hard-shell hydropropyl methylcellulose (HPMC) capsule. The HPMC capsules are gelatin-free and standard type used for medicinal products. Their function is to transport the markers to the stomach, where they dissolve. The markers pass through the gastrointestinal tract and are excreted in the feces.
The use of radiopaque markers impregnated with Barium Sulphate to measure colonic transit time has been in practice for over 45 years. Barium Sulphate is a widely used radiopaque contrast agent for various diagnostic procedures, including X-ray imaging. The term “radiopaque” refers to materials such as barium sulphate that are dense enough to resist X-rays shining through them, and so can be seen on an X-ray. Silicone is also a commonly used material in many medical technology applications.
The HPMC capsule (size -00-) is composed of two open-ended cylinders, a cap and a body, that fit inside each other. Five of the capsules contain ten ring-formed markers, while two capsules contain five tube-formed markers. The capsules are packed in a blister pack labeled with numbers, which is then placed inside a carton box.
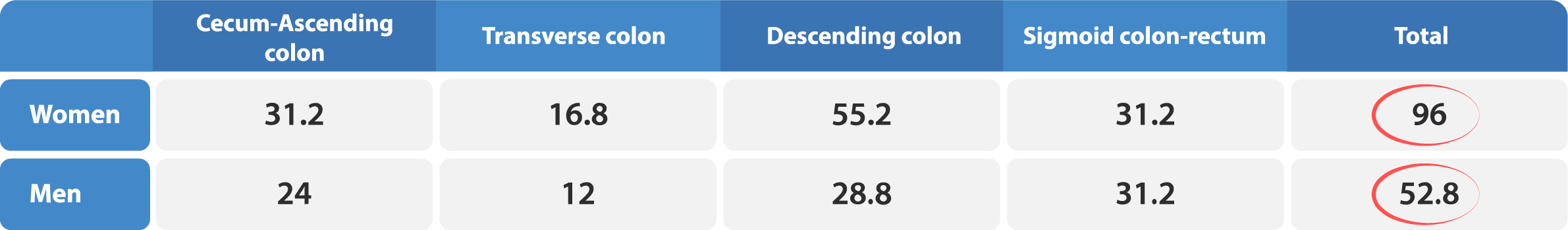
The transit times obtained are independent of the shape of the markers, however, on day six, differently shaped markers are used to aid the radiologist in identifying the location of the cecum. Additionally, dividing the day six dosage into morning and evening doses will increase the accuracy of measurements related to rapid transit.
Transit-Pellets Instruction for Use
Each box of Transit-Pellets includes an Instruction for Use (IFU), which is available in up to 20 different languages. It is important that the user of the Transit-Pellets device reads this information before beginning use of the device. The IFU contains important instructions on how to properly use the Transit-Pellets. By thoroughly reviewing the IFU, the user can ensure that they are using the Transit-Pellets safely and effectively. You will find it in the footer menu “Downloads” or you can enter it directly via THIS LINK.
A Guide to Initial Steps
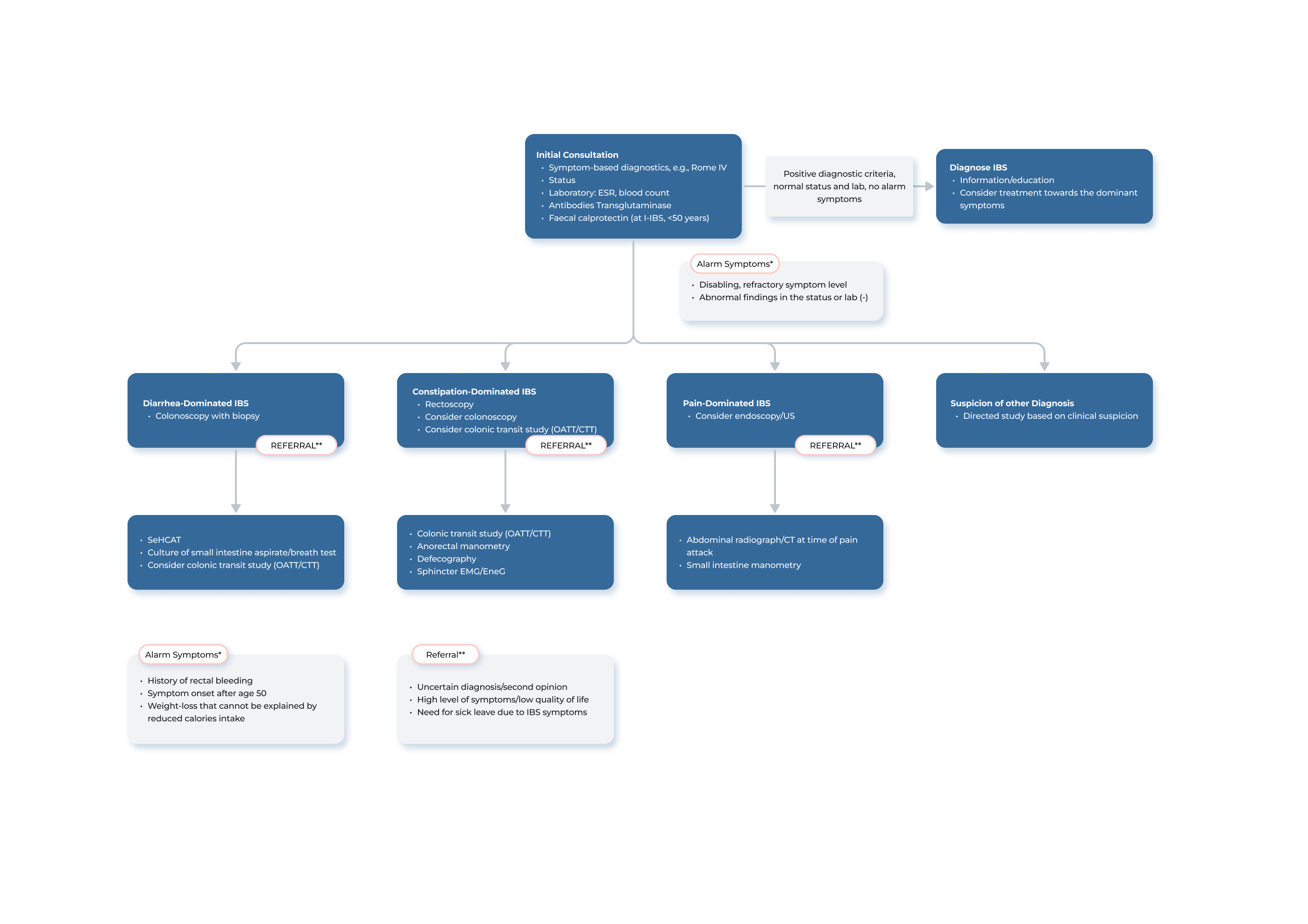
When it comes to determining the timing for colonic transit time measurements in relation to treatment steps, there may be variations in approach depending on the individual physician and medical center. However, in general, initial management of constipation often includes making dietary and lifestyle changes, as well as trying over-the-counter laxatives such as bulk-forming agents and non-absorbable carbohydrates. If these methods prove to be ineffective, a colonic transit study using Transit-Pellets radiopaque markers may be conducted. Some healthcare providers may also try other treatment options, such as saline laxatives or motility-stimulating drugs, before recommending a colonic transit test.
The Transit-Pellets are prescribed by a physician for use by the patient in the home setting. There are various ways for patients to obtain the Transit-Pellets device. It may be given to them at the clinic, mailed to them, or available for pick-up at a pharmacy.
Develop prescription pad, X-ray and Order Form.
Step-by-Step Instructions for Proper Usage of the Transit-Pellets Device
For six consecutive days, the patient ingests ten Transit-Pellets radiopaque markers contained in seven HPMC capsules followed by an abdominal radiograph on day seven.
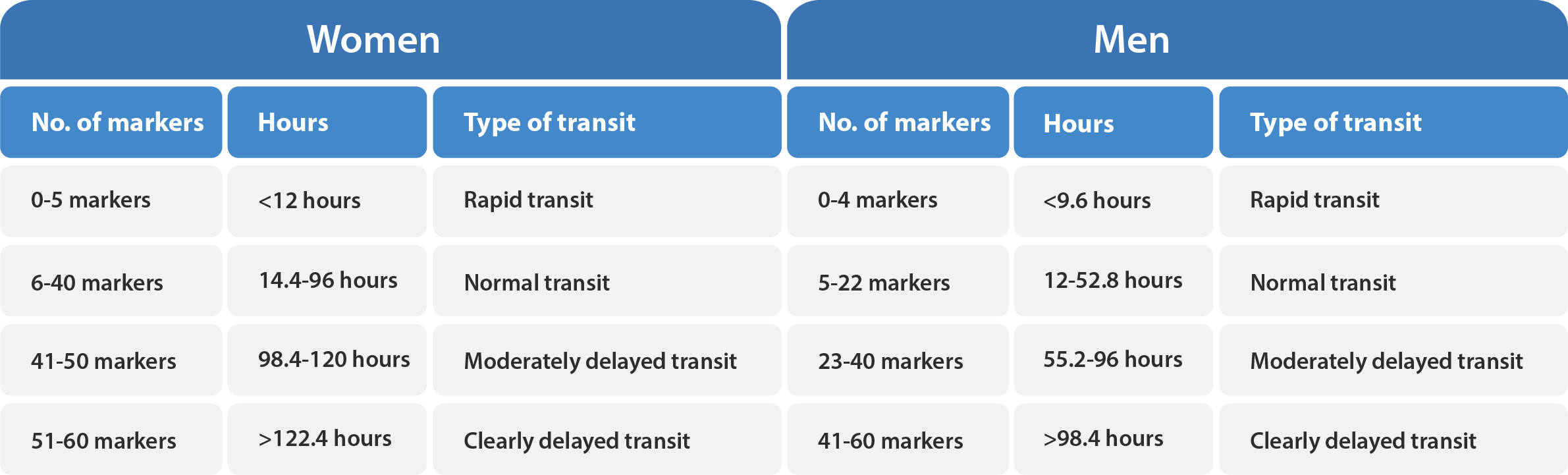
It is important to follow the prescribed dosing instructions for the markers to ensure accurate results. One capsule should be taken each day from day one to day five. On day six, one capsule should be taken in the morning, labeled as ”6:1” and one capsule should be taken in the evening, labeled as ”6:2”. To ensure the accuracy of the measurement, it is important to schedule the radiology examination at the appropriate time relative to the last marker dose on day six, labeled as ”6:2”. This means that the exam should be scheduled approximately 12 hours after the last marker dose was ingested in the evening on day six. The following table provides a helpful reference:
Ny tabell?
Supplementary Recommendations for Usage of the Transit-Pellets Device
To ensure proper and safe usage of the device, it is important to thoroughly review the Transit-Pellets Instruction for Use (IFU) prior to initiating use. You can enter it directly via THIS LINK. Please be aware that the Transit-Pellets IFU is subject to change. The most current version is always available on this site.
For individuals who cannot swallow capsules, the contents can be emptied into soft food such as applesauce or yogurt and ingested that way. Capsules and markers should not be chewed.
It is advisable for individuals to maintain their normal dietary and fluid intake during the week when ingesting Transit-Pellet markers. It is important to avoid the use of laxatives or other medications that may affect colonic motility during the testing period, as these may interfere with the accuracy of the results. It is essential to follow any instructions by the prescribing physician to guarantee the success of the test.
Bristol Stool Chart
It is recommended that patients maintain a diary of their bowel movements during the week when they are scheduled to undergo a colonic transit test with Transit-Pellets radiopaque markers. The Bristol Stool Form Scale (BSFS) is a validated tool (Aziz) that can be used to assess the consistency of stools on a spectrum of seven types and may be helpful in this regard. By comparing the patient´s self-reported symptoms, bowel movement frequency, and the objective measurements of the colonic transit test with Transit-Pellets, the physician can gain a better understanding of the patient´s problem and the results of the test in combination with the diary can be useful in clinical practice.