The Importance of Selecting Appropriate Investigation Method
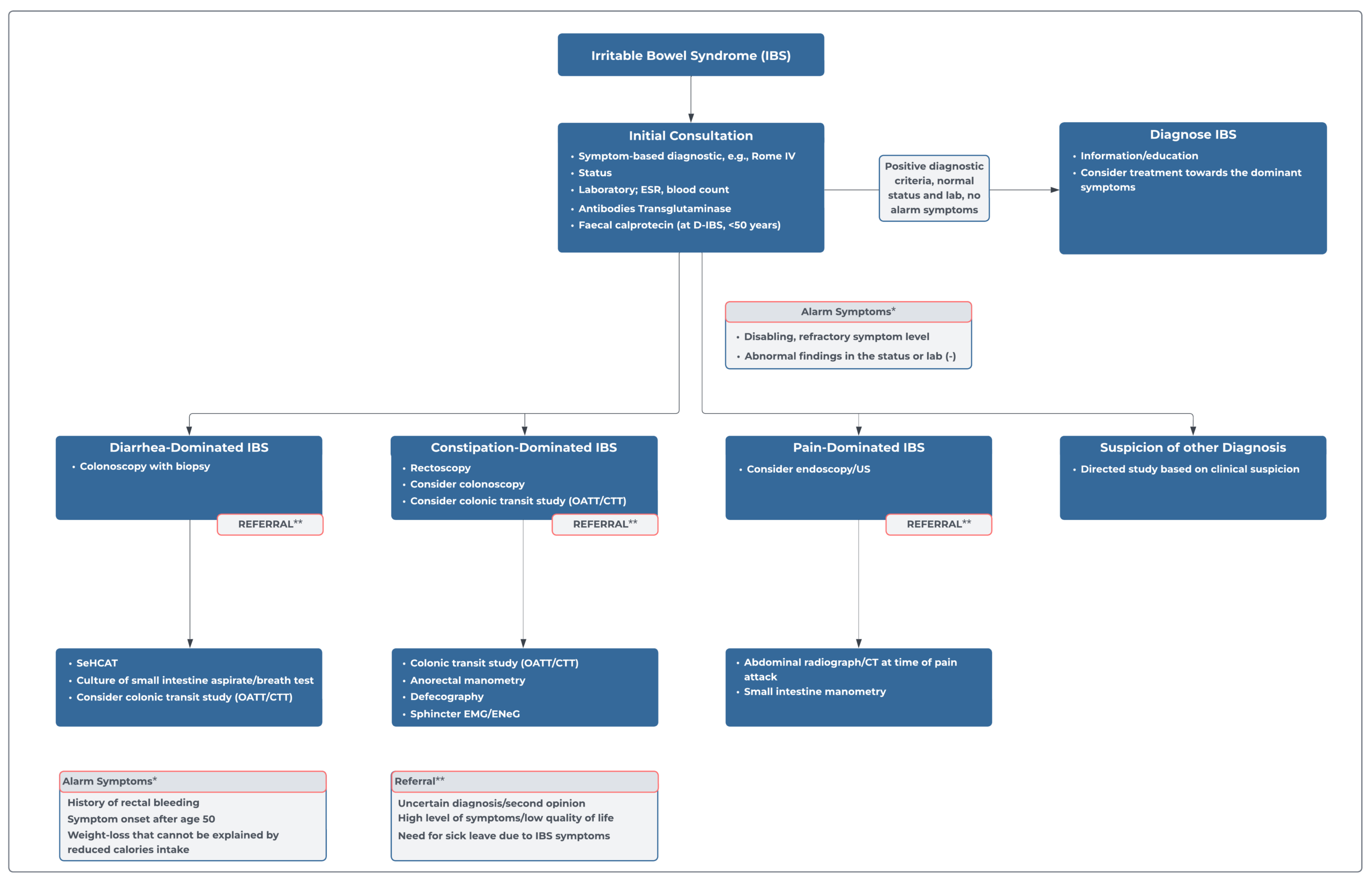
Gastrointestinal dysfunction is often not reflected in the symptoms reported by patients, highlighting the importance of conducting testing to guide treatment. Radiopaque marker studies are an effective method for measuring colonic transit time (CTT) and is widely regarded as the most reliable method for evaluating various gastrointestinal disorders in both adults and children. It is considered the gold standard by many gastroenterologists. The results obtained from this technique are highly dependable, but the accuracy of the results may vary depending on the specific technique used. Therefore, it´s important to choose the right technique to ensure the most accurate results.1
The Transit-Pellets method, formerly known as the Abrahamsson method, is a validated method and provides precise measurement of total and segmental colonic transit time. This method is a simple and cost-effective way to measure slow, normal, and rapid colonic transit. It involves the ingestion of Transit-Pellets radiopaque markers in multiple capsules over a set period of time, followed by a single abdominal X-ray on day seven.2, 3, 4
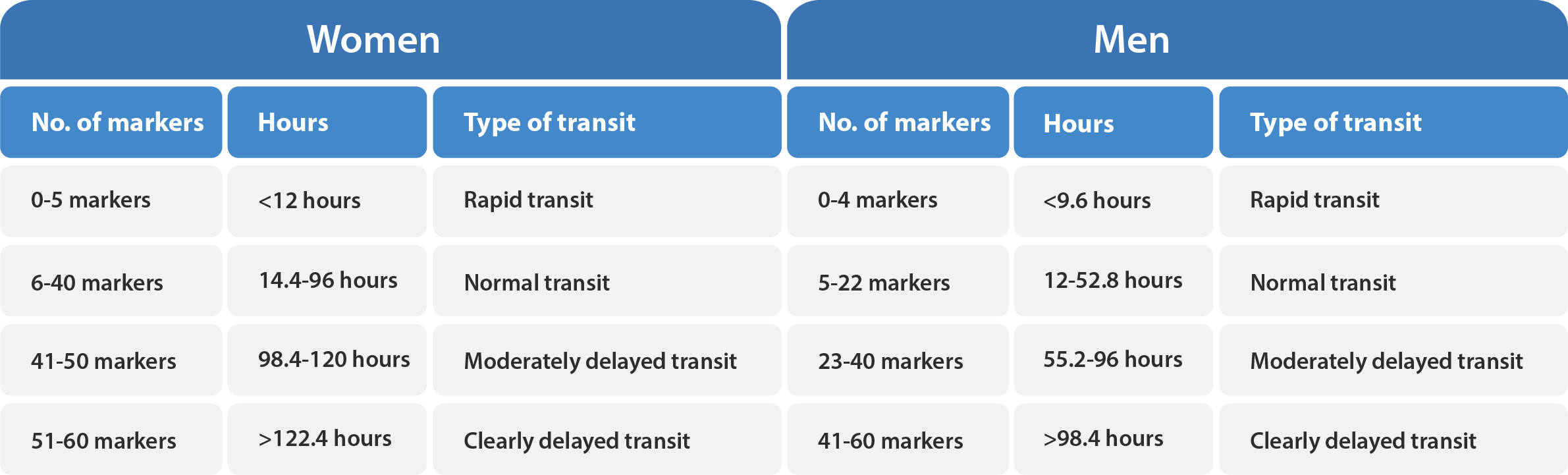
In contrast, the single marker dose technique is a qualitative method and involves the ingestion of radiopaque markers, usually in a capsule, on day one, followed by an abdominal X-ray on day five or six. The test does not provide numerical values for CTT in days or hours but use the retention of a defined fraction of the ingested markers to differentiate between normal and slow colonic transit. 5, 6, 7
Physicians and radiologists prescribing the Transit-Pellets device designed and packaged to be compatible with the Transit-Pellets method allows for a low radiation exposure while still providing numerical values for slow, normal and rapid CTT and for segmental transit times. 4
An Accurate and Reliable Method for Measuring Colonic Transit Time
It is essential that standardized, validated study protocols are followed when performing tests of gastrointestinal function.1 A colonic transit test utilizing the Transit-Pellets method and Transit-Pellets radiopaque markers can quantify the severity of transit problems, and the results can be important in determining the need for other examination procedures, selecting the appropriate therapy, and predicting long-term prognosis. The test results can be used to guide decision-making in these areas.
The Transit-Pellets method and Transit-Pellets device can be used to:
- Measure rapid, normal and slow colonic transit 8, 9
- Differentiate between slow transit and normal transit constipation 2, 10, 11
- Identify segmental colon dysfunction in patients with constipation 2
- Differentiate between normal and rapid transit diarrhoea 11, 12
- Identify treatment effects in patients with chronic constipation 13
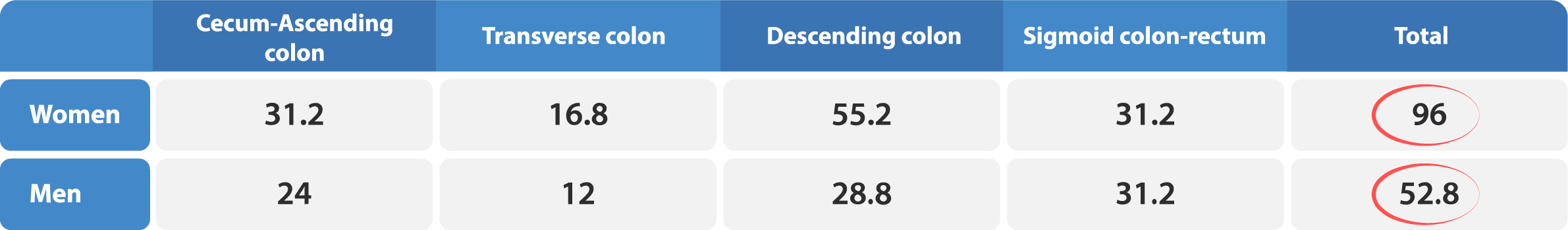
Gender Difference in Colonic Transit
Healthy females generally tend to have a notably slower intestinal transit compared to males.2, 4, 8, 14 In light of this, it is essential to take into account factors such as gender in the selection of a method for measuring colonic transit time with radiopaque markers. The Transit-Pellets method is designed to provide accurate measurement of colonic transit time, taking into account gender-specific variations which is based on robust reference values.2, 8, 15
Accurate Measurement of Rapid Colonic Transit
The Transit-Pellets method and Transit-Pellets device allows for the measurement of rapid transit, which is of significance in certain populations experiencing rapid transit diarrhoea. This is possible due to the short interval of 12 hours between the final marker intake on the sixth day and the subsequent X-ray taken on the seventh day. This schedule serves as the foundation for determining the lower limit of normal transit (the 5th percentile) and identifying cases of rapid transit.4, 11, 15
Hasse Abrahamsson
MD; Emeritus Professor of Gastroenterology.
Founder of the method in 1985.
A Brief History of the Transit-Pellets Method
The original Transit-Pellets method, formerly known as the Abrahamsson method, involves patients swallowing ten ring-formed radiopaque markers for six days, with a single abdominal radiograph taken on the seventh day to differentiate between rapid, normal, and prolonged transit. In the 1990s, studies at the Sahlgrenska Gastroenterology Section on the pathophysiology of functional and other diarrhoeal disorders prompted a modification of the method in order to measure rapid transit with enhanced precision. The modified method involves dividing the marker dose on the sixth day into two doses taken 24 and 12 hours before the X-ray on day seven. The current Transit-Pellets device, designed and packaged to be compatible with the Transit-Pellets method enables the measurement of normal and prolonged transit, as well as identifying patients with accelerated transit with enhanced precision.4 This sets it apart from other products (radiopaque markers) on the market, which do not offer this capability.
If you wish to gain further insight into the evolution of the Transit-Pellets method and Transit-Pellets radiopaque markers, we recommend visiting the section entitled Origin and Development of the Transit-Pellets Method.
- Keller, J., Bassotti, G., Clarke, J., et al. (2018). EXPERT CONSENCUS DOCUMENT. Advances in the diagnosis and classification of gastric and intestinal motility disorders [electronic version]. Nat Rev Gastroenterol Hepatol., Vol. 15(5), 291-308.
- Abrahamsson, H., Antov, S. & Bosaeus, I. (1988). Gastrointestinal and colonic segmental transit time evaluated by a single abdominal X-ray in healthy subjects and constipated patients. Scand J Gastroenterol. Vol. 23 (suppl 152), 72-80.
- Aziz, I., Whitehead, W.E., Palsson, O., Törnblom, H. & Simrén, M. (2020). Expert Review of Gastroenterology & Hepatology. An approach to the diagnosis and management of Rome IV functional disorders of chronic constipation [electronic version]. Taylor & Francis Group, Vol. no. 14, 1-8.
- 0020 Clinical Evaluation Report – Transit-Pellets ver08 (Internal Document). November 2023.
- Kim, E.R. & Rhee, P-L., (2012). How to Interpret a Functional or Motility Test – Colon Transit Study. J Neurogastroenterol Motil., Vol. 18(1), 94-99.
- Keighley, M. & Williams, N. (2018). Keighley & Williams´ Surgery of the Anus, Rectum and Colon. 1664 pages Boca Raton: CRC Press.
- Popescu, M. & Mutalib, M. (2021). Bowel transit studies in children: evidence base, role and practicalities. Frontline Gastroenterol., Vol. 13(2), 152-159.
- Sadik, R., Abrahamsson, H., Stotzer, P.O. (2003). Gender differences in gut transit shown with a newly developed radiological procedure. Scand. J. Gastroenterol., Vol. 38, 36-42.
- Abrahamsson, H. & Antov, S. (2010). Accuracy in assessment of colonic transit time with particles: how many markers should be used? Neurogastroenterol Motil., Vol. 22, 1164-69.
- Törnblom, H., van Oudenhove, L., Sadik, R., Abrahamsson, H., Tack, J., Simrén, M. (2012). Colonic transit time and IBS symptoms: what’s the link? Am. J. Gastroenterol., Vol. 107(5), 754-60.
- Abrahamsson, H., Ostlund-Lindqvist, A.M., Nilsson, R., Simrén, M., Gillberg, P.G. (2008). Altered bile acid metabolism patients with constipation-predominant irritable bowel syndrome and functional constipation. Scand J Gastroenterol., Vol. 43(12), 1483-8.
- Sadik, R., Abrahamsson, H., Ung, K.A., et al. (2004). Accelerating regional bowel transit and overweight shown in idiopathic bile acid malabsorption. Am. J. Gastroenterol., Vol. 99, 711-718.
- Simrén, M., Bajor, A., Gillberg, PG., Rudling, M., Abrahamsson, H. (2011). Randomised clinical trial: The ileal bile acid transporter inhibitor A3309 vs. placebo in patients with chronic idiopathic constipation-a double-blind study. Aliment Pharmacol Ther., Vol. 34(1), 41-50.
- Disney, B., et al. (2019). Cost of Constipation Report. https://bowelinterestgroup.co.uk/wp-content/uploads/2019/07/The-Cost-of- Constipation-2019.pdf. Acceded on January 24, 2023.
- Törnblom, H., et al. Data on File, Gastrointest Lab, Sahlgrenska University Hospital.