About Medifactia AB
Medifactia manufactures and distributes radiopaque markers products, offering top-notch quality, providing superb performance and functionality at affordable cost. The company is ISO 13485 certified, holds CE mark, FDA and TGA clearance for its radiopaque marker’s device. Transit-Pellets radiopaque markers offer the most reliable patient friendly and cost-effective colon transit markers.
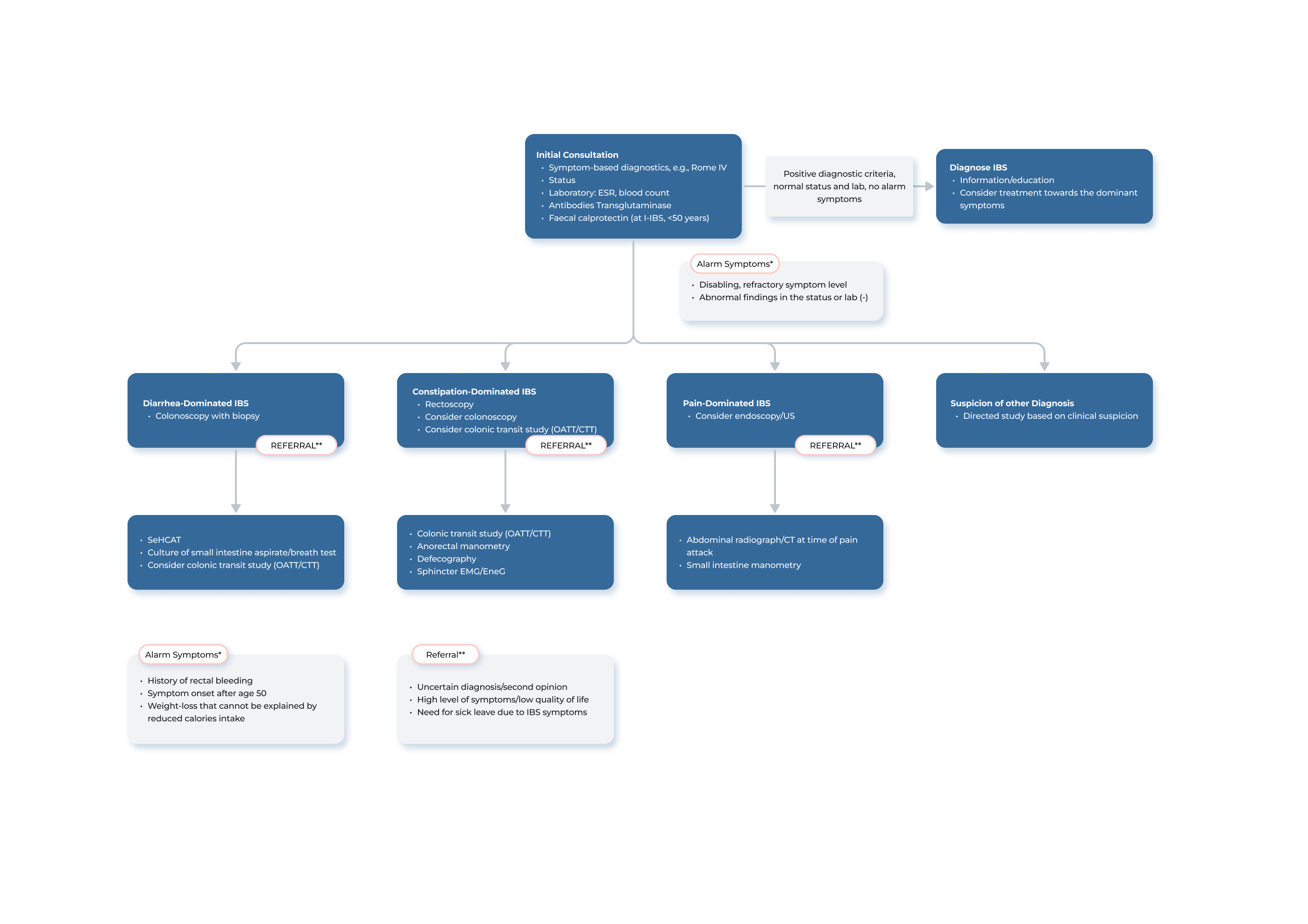
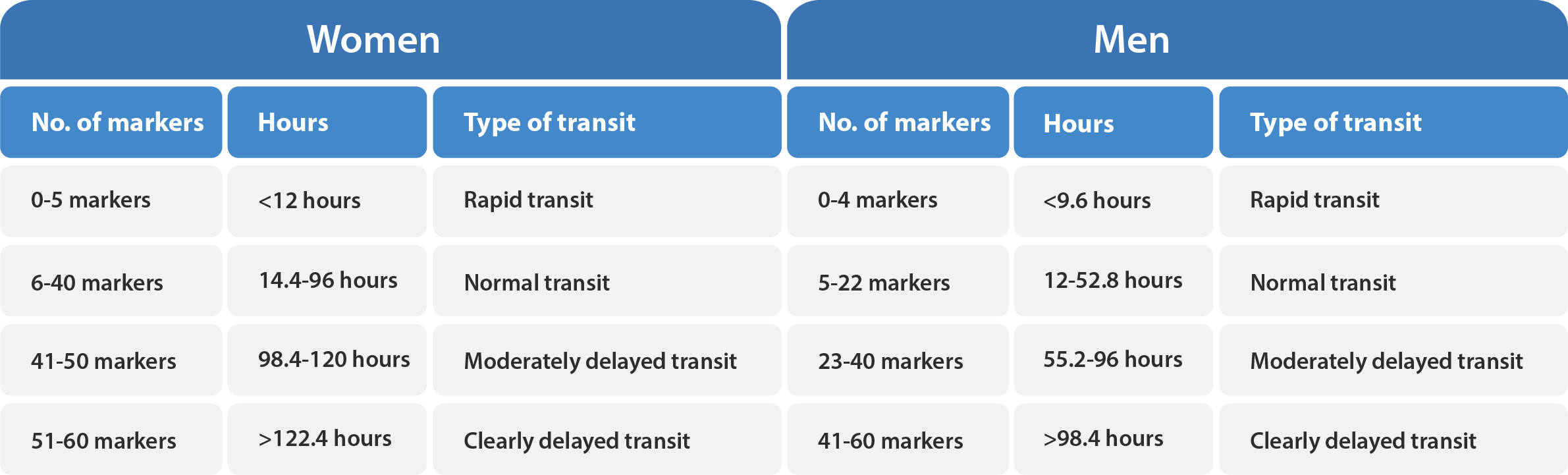
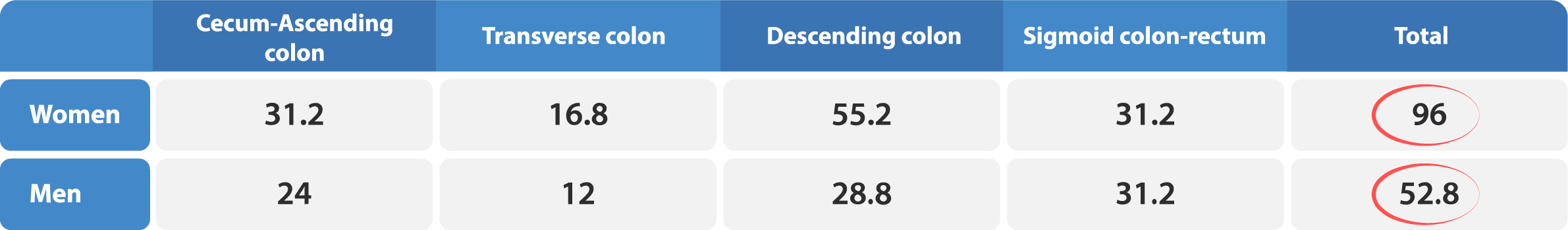
Transit-Pellets radiopaque markers offer the latest state of the art options for colon transit tests. This test is known by a few names – Colon Transit-Pellets test, colonic transit markers study, radiopaque markers test, Sitz marker study, or a radio-opaque markers test. The device is manufactured and packed according to Transit-Pellets method (Formerly known as: Abrahamsson’s Method).
A unique, and the validated method by which to measure the whole spectrum of colon transit time (CTT), from rapid to slow transit values.
In addition, the company offers customers of Transit-Pellets radiopaque markers access to World’s Premiere online standardized reporting system for radiologists to treating physicians.
Medifactia’s Transit-Pellets complete system offerings for colon transit tests with radiopaque markers is becoming a benchmark of clinical excellence and is features in many published works by researchers around the world.
We are ISO 13485 Certified!
The ISO 13485:2016 specifies the requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer and applicable regulatory requirements.