Sundhedspersonale
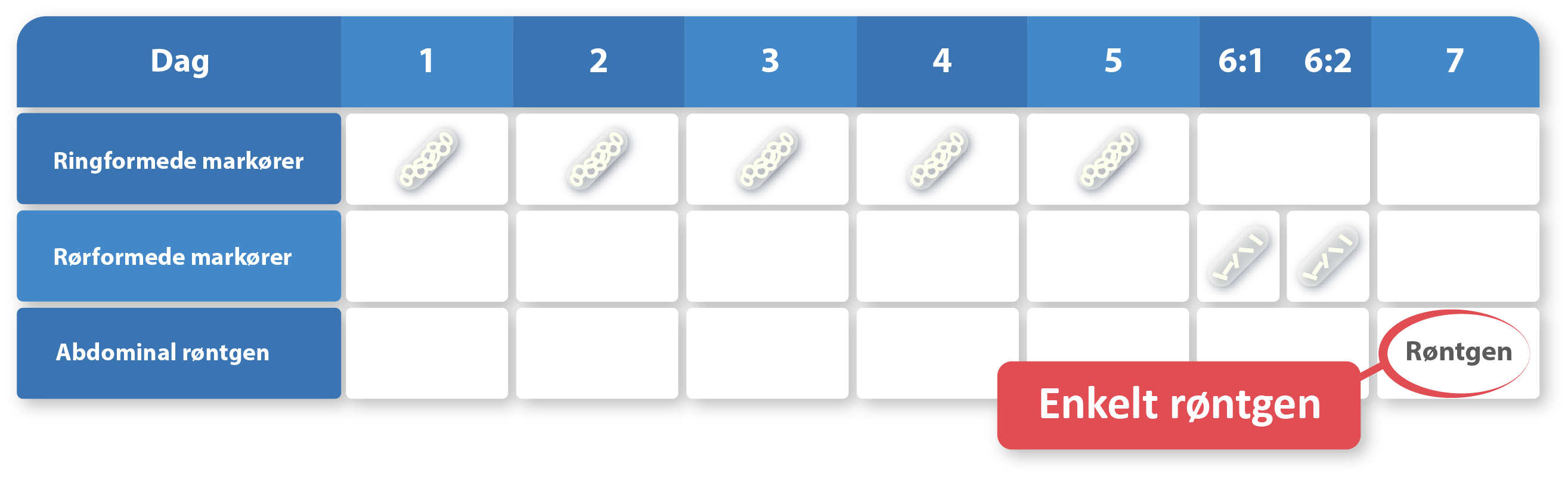
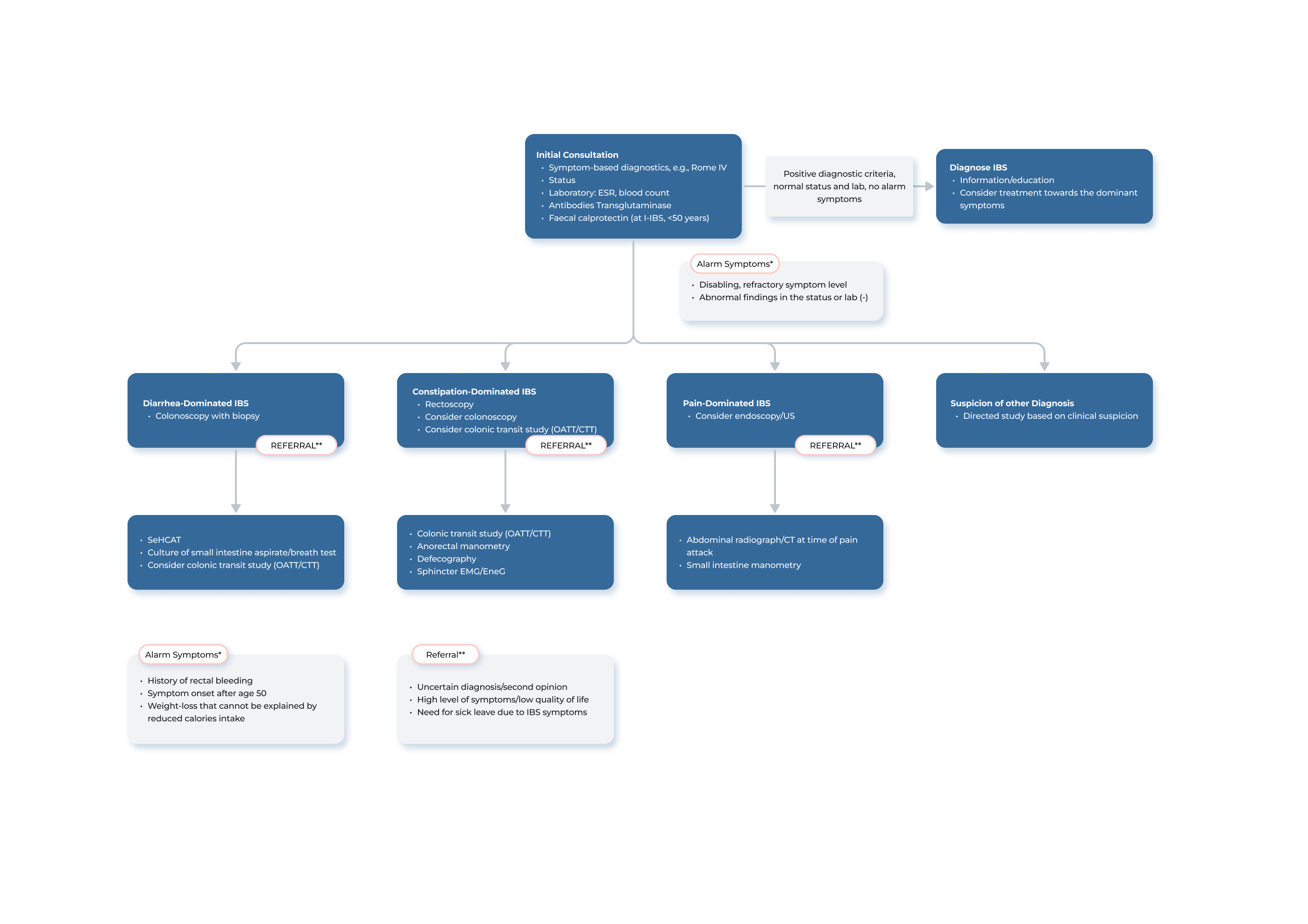
Vi er stolte af at tilbyde state-of-the-art Transit-Pellets røntgen markører, der er designet til at forbedre vurderingen og behandlingen af motilitetsforstyrrelser som forstoppelse, diarré og irritabel tyk tarm (IBS). Vores markører er fremstillet i henhold til Transit-Pellets method. Derudover leverer vi en online standardiseret kolontransit-testrapport, der er designet til behandlende læger og radiologer.
Valideret metode og sikre colon transit røntgen markører til kvantitativ vurdering af den samlede og regionale tyktarmstransit
Transit-Pellets method og Transit-Pellets radio-uigennemsigtige markører (radiopaque markers) kan anvendes til følgende:
- at måle hurtig, normal og langsom tyktarmstransit
- at skelne mellem langsom transit og normal transitforstoppelse
- at identificere segmentopdelt tyktarmsdysfunktion hos patienter med forstoppelse
- at skelne mellem normal og hurtig transitdiarré
- at identificere behandlingseffekter hos patienter med kronisk forstoppelse
En tyktarmstransittest med Transit-Pellets method, tidligere kendt som Abrahamssons method, og Transit-Pellets røntgen markører kan kvantificere transitproblemernes alvorlighed. Testen kan være vigtig for at bestemme behovet for yderligere undersøgelsesprocedurer, vælge den passende behandling og fastsætte en langfristet prognose. Testresultaterne kan anvendes som hjælp til beslutningstagning på disse områder.
Der kan findes henvisninger i afsnittet VALIDATED METHOD.
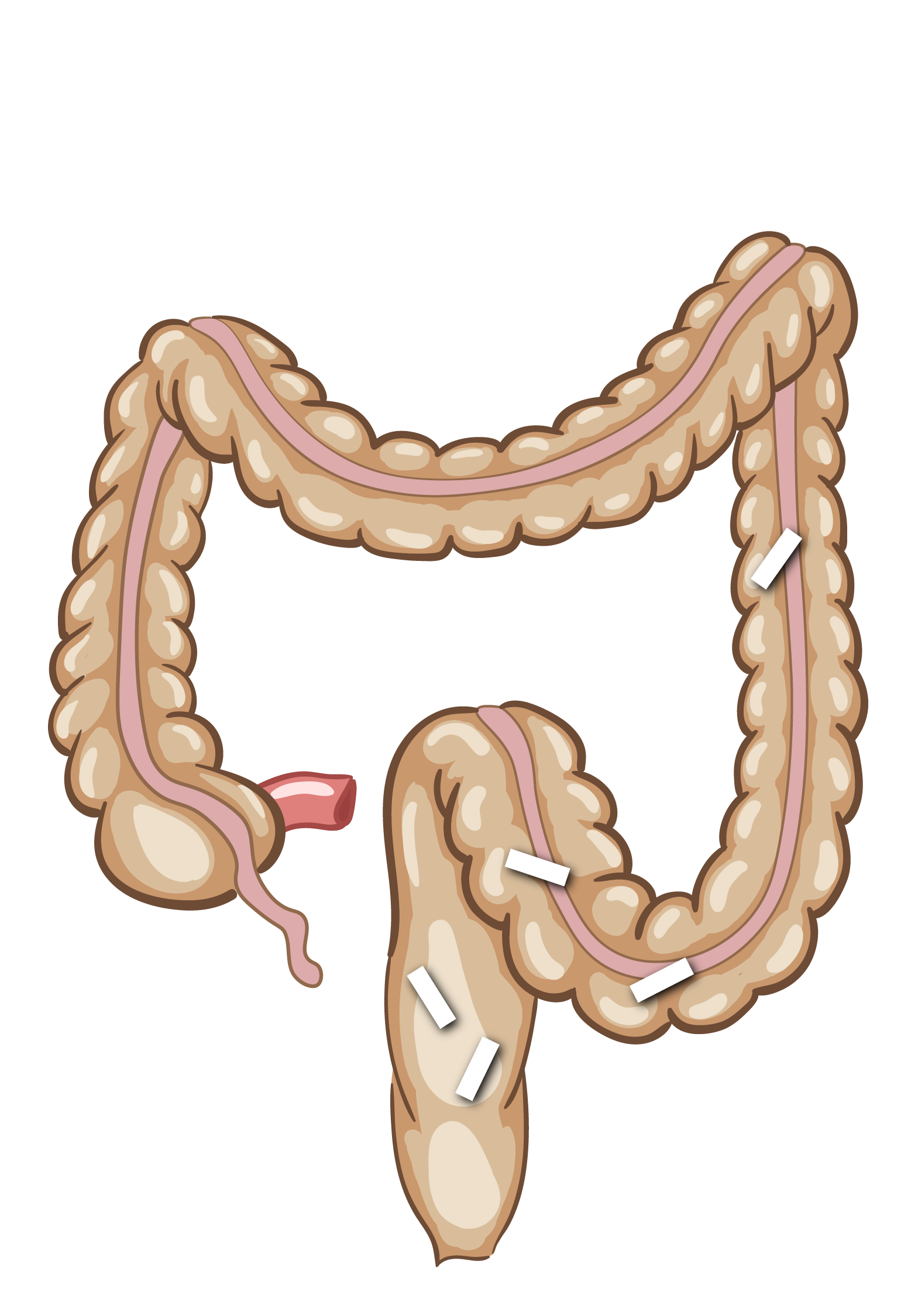
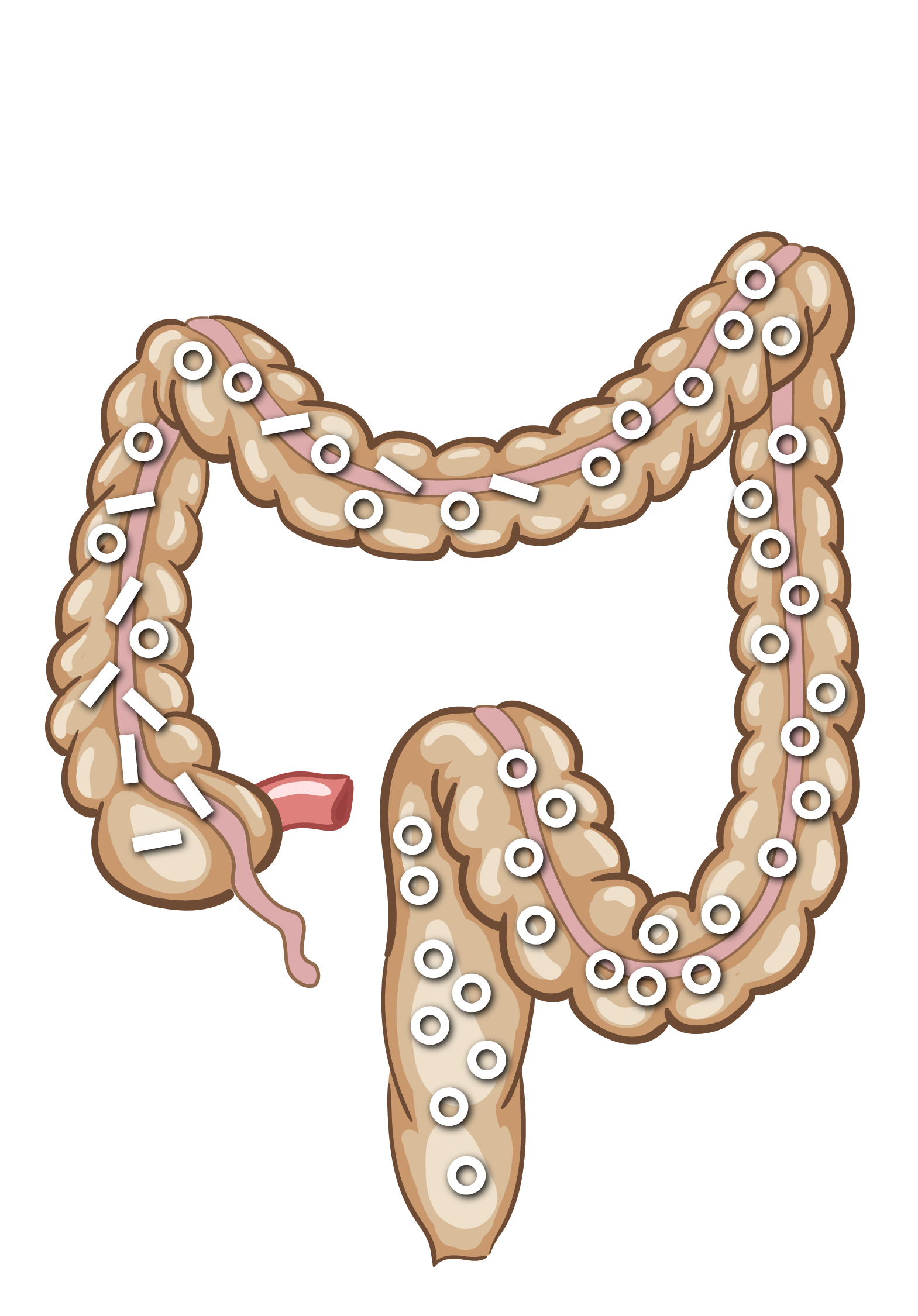
Illustration af Transit-Pellets-markørbevægelse

Questions?
Contact Us. We’re here to help!
Diana Nyström
+46 (0) 8-460 072 06
diana.nystrom@medifactia.com